Core Research Projects
Characterizing the spatiotemporal dynamics of microglia-driven synapse remodeling in vivo
The phagocytic engulfment of synapses is the predominant method through which microglia are thought to regulate synaptic connectivity in the brain. However, defining the detailed structural events through which microglia phagocytose synapses has been challenging due in large part to limitations in the spatial and temporal resolution of two-photon microscopy. Moreover, emerging evidence from our lab suggests that microglia can also shape synapses through non-phagocytic mechanisms that are not well-understood, particularly during developmental phases of experience-dependent synaptic refinement.
To systematically define the structural and functional responses of microglia to sensory stimulation in vivo, we visualize and quantify interactions between microglia and synapses in live, awake mice as the animals are exposed to diverse visual stimuli. To overcome the technical challenges that have thus far hampered attempts to fully define the spatiotemporal dynamics of microglia-synapse interactions, we have built a two-photon microscope capable of rapidly imaging three fluorescent channels at one time and are designing molecular tools (e.g. ratiometric and FRET-based probes) for detecting interactions between microglia and synapses at nanometer resolution. Through these approaches, we are uncovering fascinating new insights into the roles of these brain-resident immune cells in the experience-dependent remodeling of functional synaptic connections.
Profiling the roles of microglia in visual feature detection and computational processing
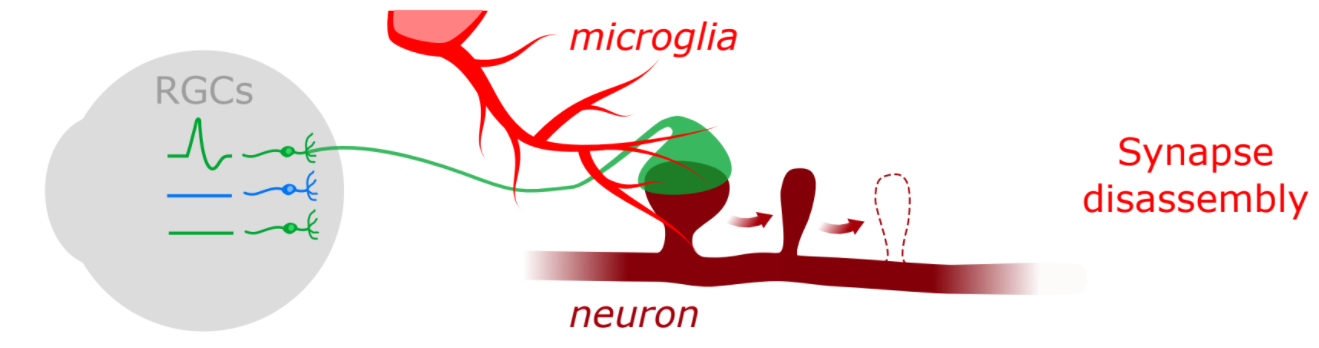
Schematic of retinocollicular outcomes
Synapses are not only loci of information transfer between neurons but also key points of information processing in the brain. Given recent evidence that microglia possess the ability to interpret and respond to local changes in synaptic activity, one question that has arisen is whether microglia are active players in neural computation. We hypothesize that microglia can go beyond removing synapses based upon local activity signals to detect and interpret differences in the patterning of activity at synapses, which may allow microglia to interact differently with synapses depending upon the unique features of the visual world that they encode. To test this hypothesis, we are using transgenic and viral approaches to label functionally distinct classes of RGCs and imaging interactions between these RGCs’ synapses and microglia in the superior colliculus. Ultimately, we will expand these studies to identify the molecular mechanisms that enable microglia to interact with synapses in a functionally-specific manner.
Identifying the genetic regulatory machinery that controls experience-dependent transcription in microglia
Schematic of microglia and nucleus
One of the most fascinating findings to emerge from our laboratory is the observation that microglia mount robust transcriptional responses to visual stimulation. We hypothesize that these experience-dependent changes in chromatin accessibility and transcription are coordinated by dedicated genetic regulatory factors in the microglial nucleus. To identify these factors and understand how they coordinate inducible transcription in microglia, we acutely isolate microglia from the cortices of sensory deprived or stimulated mice and interrogate (1) transcription using single-cell and bulk RNA-sequencing; (2) chromatin accessibility using ATAC-sequencing; and (3) histone modifications and transcription factor binding using CUT&RUN. It is our ultimate goal to identify the transcriptional regulatory factors that drive sensory-dependent gene expression in microglia, and to harness these genome-wide datasets to develop new mouse lines in which stimulated microglia can be visualized and manipulated experimentally.
In addition to applying these cutting-edge approaches to mice, we also analyze inducible transcription in all cell types of human cortex following electrical stimulation in vivo. This complementary approach allows us to explore the extent to which genetic mechanisms identified in mice are evolutionarily conserved in the human, and has the potential to identify human-specific mechanisms relevant to neurological disease.
Determining how impairments in neuro-immune communication contribute to disorders of the human brain
As a basic science laboratory, our research is motivated by an intense curiosity about the mechanisms that shape the developing brain. But ultimately, we are interested in harnessing the mechanistic insights we derive in the mouse to shed light on the causes of human brain disorders arising across the lifespan, from autism to Alzheimer’s disease. Toward this end, we take advantage of existing mouse models, and seek to develop new and improved models, to explore how impairments in the process of microglia-driven synapse remodeling contribute to neuropathology. In parallel, we derive translational insight by analyzing the transcriptional changes that occur in human brain cells in epilepsy, a debilitating disorder that is prevalent in individuals with conditions like autism and schizophrenia.
The Big Picture
By applying a multidisciplinary strategy to the brains of mice and humans, we are making major discoveries about how neurons and immune cells communicate to shape the brain. In addition to becoming leaders in the fields of neuroimmunology and developmental neuroscience over the coming years, we strive to establish a uniquely dynamic and collaborative research program by interfacing with other investigators both within our discipline and beyond. Ultimately, we aim to transform our growing understanding of experience-dependent refinement and plasticity in the healthy brain into new therapeutic strategies for treating neurodevelopmental, psychiatric, and neurodegenerative disorders.